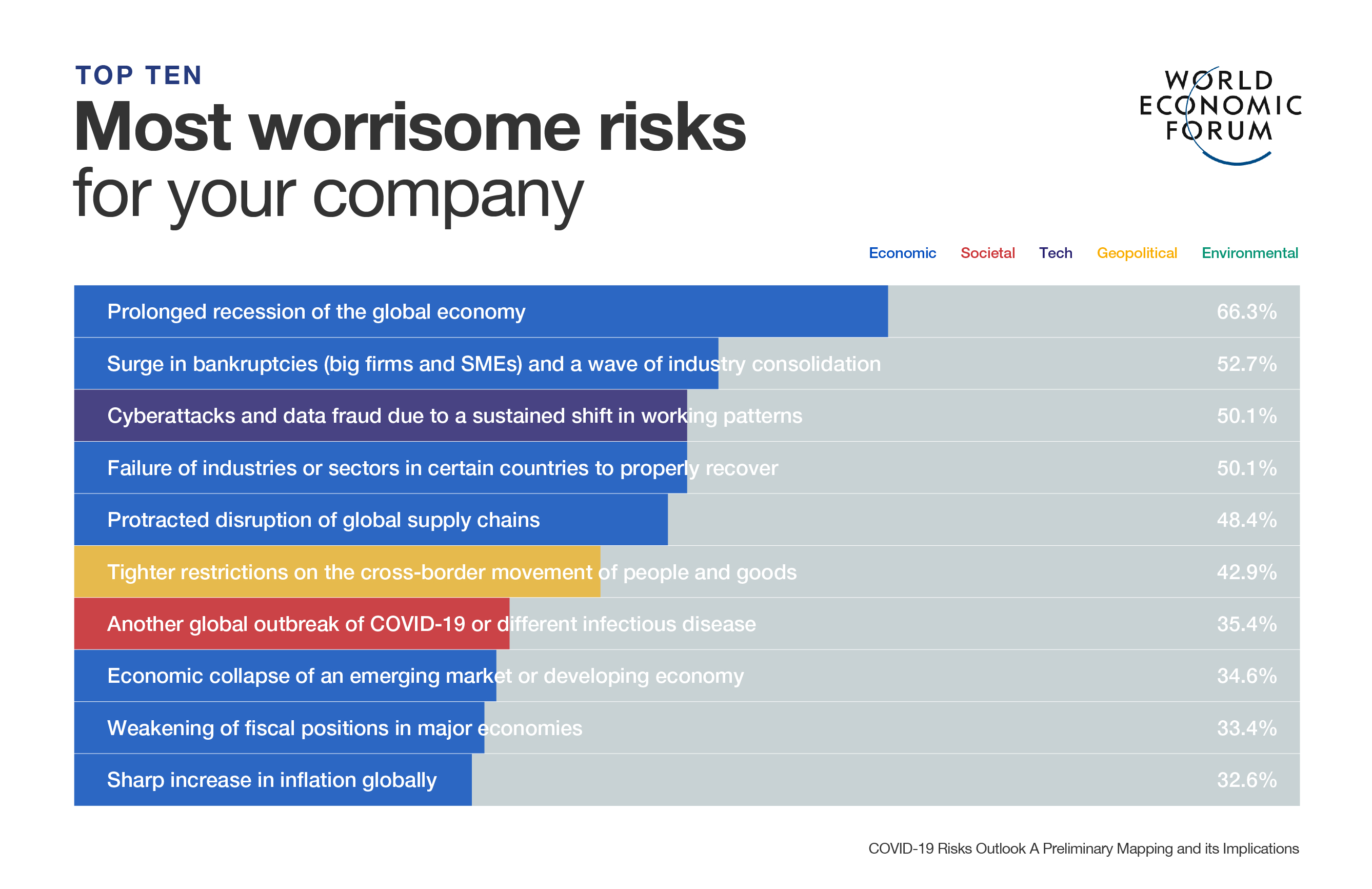
Why this sea creature is still a key ingredient in testing vaccines
There are about 400 coronavirus drugs and vaccines in development, all need to be tested using horseshoe crab blood.
Image: REUTERS/Mike Segar
Explore and monitor how COVID-19 is affecting economies, industries and global issues
Stay up to date:
COVID-19
- For decades, horseshoe crab blood has been instrumental in making sure vaccines are safe.
- Calls for a man-made alternative to be used have been dealt a blow by an influential US standards group.
- All injectable medicines, including any potential COVID-19 vaccine, must be tested for harmful endotoxins.
The milky-blue blood of an invertebrate older than the dinosaurs might not be front of your mind when rolling up your sleeve for an injection.
So it may come as a surprise to learn this exact ingredient is instrumental in the testing of vaccines around the world.
Hundreds of thousands of horseshoe crabs are caught for this purpose every year in fact. And while the miracle work of these creatures might usually fly under the radar, it has hit the headlines as the race to find a COVID-19 vaccine ramps up.
With about 400 coronavirus drugs and vaccines in development and safety tests using horseshoe crab blood currently the industry standard, this unusual-looking critter is set to play an important role in the pandemic.
Living fossils
Horseshoe crabs are commonly fished off the East Coast of the United States for use in medicine.
They’re not actually crabs, rather arthropods more closely related to scorpions, and they have existed nearly unchanged for more than 440 million years. Due to this ancient lineage, they are often referred to as “living fossils”.
One of the reasons the species has survived for so long lies in that blue blood, so coloured because it’s rich with copper. It clots when it encounters bacteria, making it both a lifesaver to the horseshoe crab and essential to human medicine today.
What is the World Economic Forum doing to manage emerging risks from COVID-19?
Crucial test
Since the late 1970s, the clotting agent in the Atlantic horseshoe crab’s blood has been used for the limulus amebocyte lysate (LAL) test, which detects bacterial endotoxins in drugs and intravenous devices. This kind of contamination could be harmful and even fatal to patients if it gets into the bloodstream.
Scientists take about a third of a horseshoe crab’s blood for use in the tests, after which the creature is released back into the ocean. Companies that make LAL tests say the animals are not harmed during the procedure.
According to some estimates, though, 15% may die as a result of the process. And there’s concern the horseshoe crab is facing pressure on many fronts – it’s also fished to be used as bait and suffering due to habitat loss and rising sea levels. It has a key role in the ecosystem, too, with its eggs providing food for bird species including the threatened red knot.
Synthetic alternative
Wildlife groups worried about these impacts have called for a man-made alternative – recombinant Factor C (rFC) – to be used instead of LAL.
The organization that issues quality standards for such tests in the States, US Pharmacopeia (USP), says rFC is more sustainable because it can be made in unlimited quantities.
But USP also believes more data is needed before it can give the new test and LAL equal status – something that Europe is moving to do. Which means that, for now, as Reuters reports, horseshoe crabs’ blood will remain the drug industry standard.
Blue gold
About 70 million endotoxin tests are performed annually in a roughly $1 billion market. And the horseshoe crab blood currently so essential to its operation is thought to be worth about $16,000 dollars a litre, according to Bloomberg – no wonder it’s sometimes referred to as blue gold.
In the coming months, and until a man-made alternative becomes more established, it could be worth its weight if it helps scientists deliver the billions of doses of a COVID-19 vaccine that will be needed around the world.
Accept our marketing cookies to access this content.
These cookies are currently disabled in your browser.
Accept our marketing cookies to access this content.
These cookies are currently disabled in your browser.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Forum Stories newsletter
Bringing you weekly curated insights and analysis on the global issues that matter.
More on Health and Healthcare SystemsSee all
Ahmed Elhusseiny
May 15, 2025
Shyam Bishen
May 13, 2025
Shyam Bishen
May 9, 2025