What’s the difference between a PCR and antigen COVID-19 test? A molecular biologist explains

PCR tests and antigen tests are the two main ways to detect SARS-CoV-2.
Image: REUTERS/Heo Ran/File Photo
Stay up to date:
Pandemic Preparedness and Response
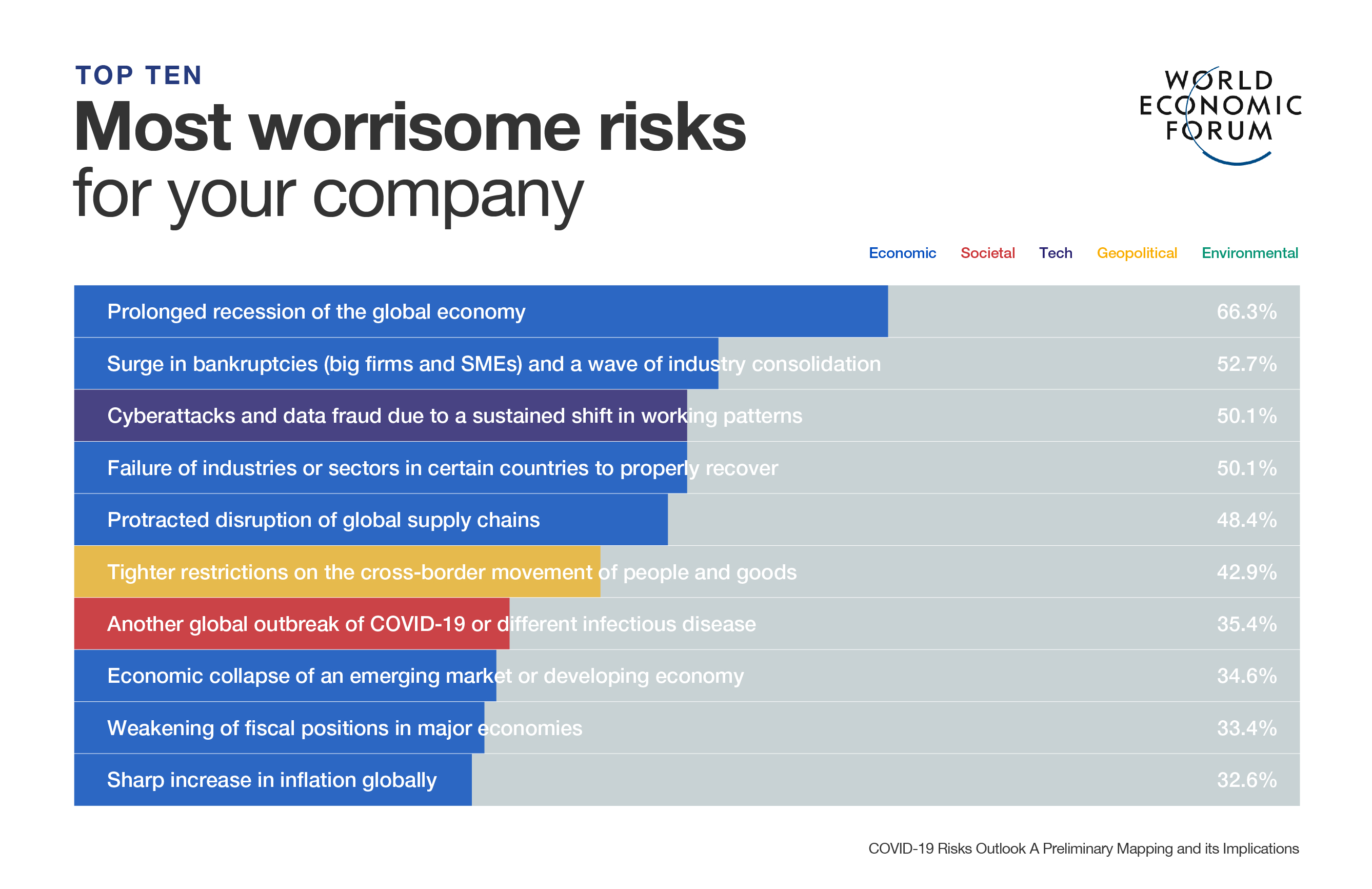
- PCR tests and antigen tests are the two main ways to detect SARS-CoV-2.
- PCR tests are nearly 100% accurate, and are analysed in a lab.
- It can take up to five days for a person to get PCR results back, costing around $100 or more per test.
- Antigen tests can produce results in less than 15 minutes and cost between $10-$15, but they are much less accurate especially during early infection.
- A professor in molecular medicine explains the science between the two.
At this point in the pandemic, you or someone you know has probably received at least one COVID-19 test. But do you know which kind of test you got and the strengths and weaknesses of these different tests?
I’m a molecular biologist, and since April 2020 I’ve been part of a team working on a National Institutes of Health-funded program called RADx that is helping innovators develop rapid tests to detect when a person is infected with SARS-CoV-2, the virus that causes COVID-19.
Two major types of tests are used to diagnose infection with SARS-CoV-2: molecular tests – better known as PCR tests – and antigen tests. Each detects a different part of the virus, and how it works influences the test’s speed and relative accuracy. So what are the differences between these types of tests?
Looking for genetic evidence
The first step for either kind of test is to get a sample from the patient. This can be a nasal swab or a bit of saliva.
For PCR tests, the next step is amplification of genetic material so that even a small amount of coronavirus genes in the patient’s sample can be detected. This is done using a technique called a polymerase chain reaction. A health care worker takes the sample and treats it with an enzyme that converts RNA into double-stranded DNA. Then, the DNA is mixed with a solution containing an enzyme called a polymerase and heated, causing the DNA to separate into two single-stranded DNA pieces. The temperature is lowered, and polymerase, with the help of a small piece of guide DNA called a primer, binds to the single-stranded DNA and copies it. The primers ensure that only coronavirus DNA is amplified. You’ve now created two copies of coronavirus DNA from the original one piece of RNA.
Laboratory machines repeat these heating and cooling cycles 30 to 40 times, doubling the DNA until there are a billion copies of the original piece. The amplified sequence contains fluorescent dye that is read by a machine.
The amplifying property of PCR allows the test to successfully detect even the smallest amount of coronavirus genetic material in a sample. This makes it a highly sensitive and accurate test. With accuracy that approaches 100%, it is the gold standard for diagnosing SARS–CoV–2.
However, PCR tests have some weaknesses too. They require a skilled laboratory technician and special equipment to run them, and the amplification process can take an hour or more from start to finish. Usually only large, centralized testing facilities – like hospital labs – can conduct many PCR tests at a time. Between sample collection, transportation, amplification, detection and reporting, it can take from 12 hours to five days for a person to get results back. And finally, they aren’t cheap at $100 or more per test.

What is the World Economic Forum doing to manage emerging risks from COVID-19?
Antigen tests
Rapid, accurate tests are essential to contain a highly contagious virus like SARS-CoV-2. PCR tests are accurate but can take a long time to produce results. Antigen tests, the other major type of coronavirus test, while much faster, are less accurate.
Antigens are substances that cause the body to produce an immune response – they trigger the generation of antibodies. These tests use lab-made antibodies to search for antigens from the SARS-CoV-2 virus.
To run an antigen test, you first treat a sample with a liquid containing salt and soap that breaks apart cells and other particles. Then you apply this liquid to a test strip that has antibodies specific to SARS-CoV-2 painted on it in a thin line.
Just like antibodies in your body, the ones on the test strip will bind to any antigen in the sample. If the antibodies bind to coronavirus antigens, a colored line appears on the test strip indicating the presence of SARS-CoV-2.
Antigen tests have a number of strengths. First, they are so easy to use that people with no special training can perform them and interpret the results – even at home. They also produce results quickly, typically in less than 15 minutes. Another benefit is that these tests can be relatively inexpensive at around $10-$15 per test.
Antigen tests do have some drawbacks. Depending on the situation, they can be less accurate than PCR tests. When a person is symptomatic or has a lot of virus in their system, antigen tests are very accurate. However, unlike molecular PCR tests, antigen tests don’t amplify the thing they are looking for. This means there needs to be enough viral antigen in the sample for the antibodies on the test strip to generate a signal. When a person is in the early stages of infection, not a lot of virus is in the nose and throat, from which the samples are taken. So, antigen tests can miss early cases of COVID-19. It’s also during this stage that a person has no symptoms, so they are more likely to be unaware they’re infected.
More tests, better knowledge
A few antigen tests are already available over the counter, and on Oct. 4, 2021, the Food and Drug Administration granted emergency use authorization to another at-home antigen test. The U.S. government is also pushing to make these tests more available to the public.
At RADx, the project I am a part of, we are currently conducting clinical studies to get a better understanding of how antigen tests perform at various stages of infection. The more data scientists have on how accuracy changes over time, the more effectively these tests can be used.
Understanding the strengths and limitations of both PCR and antigen tests, and when to use them, can help to bring the COVID-19 pandemic under control. So the next time you get a COVID-19 test, choose the one that is right for you.
Accept our marketing cookies to access this content.
These cookies are currently disabled in your browser.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Forum Stories newsletter
Bringing you weekly curated insights and analysis on the global issues that matter.
More on Health and Healthcare SystemsSee all
Shiloh Paswani and Mansoor Aamir
August 14, 2025
James Balzer
August 14, 2025
Madeleine North
August 13, 2025
Charlotte Edmond
August 11, 2025
Adriana Banozic-Tang and Heng Wang
August 5, 2025
Tom Crowfoot
July 30, 2025