Are we ready for genetically modified animals?

Genetically engineered angelfish
Image: REUTERS/Pichi Chuang
Stay up to date:
Emerging Technologies
Imagine a world with less expensive and more resilient crops, plants that can meet the world’s need for liquid biofuels, no more malaria-carrying mosquitos, real blue roses, living woolly mammoths, unicorns and a few devastating new plagues.
These fantasies may before long become realities through the use of a biomolecular tool called CRISPR/Cas9 or its descendants. CRISPR/Cas9 is the most important biological invention thus far in the 21st century: it opens the entire living world to human manipulation, far more accurately, cheaply and easily than ever before. Much ink has been spilled over the possible implications of its use in humans; far too little about humans’ possible uses of it on the rest of the biosphere.
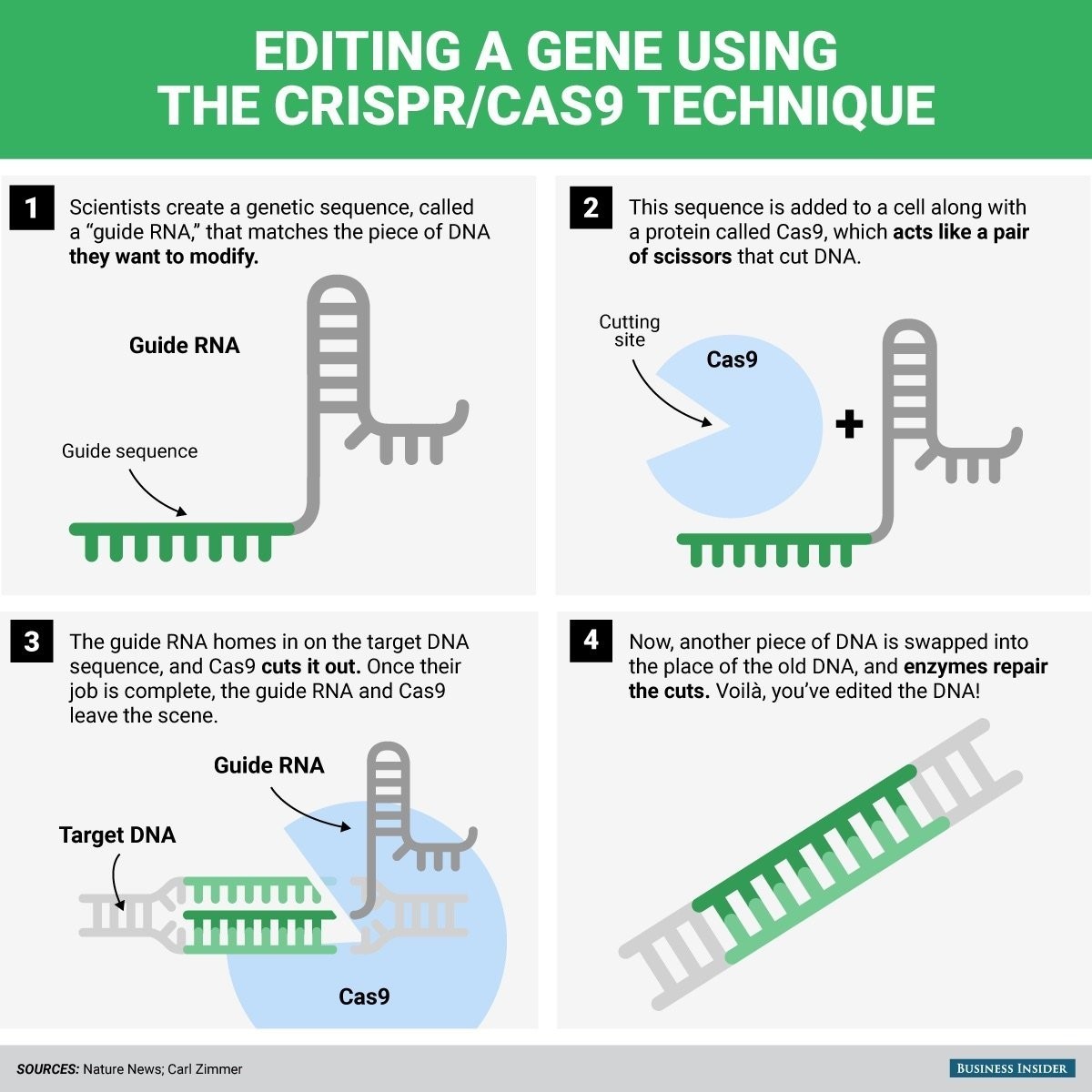
What exactly is CRISPR/Cas9?
CRISPR/Cas9 is two acronyms: Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-Associated Protein 9. Thousands of molecular complexes like it were invented billions of years ago by bacteria as protection against viruses. Researchers spent a over decade trying to understand these CRISPRs before, in 2012, Jennifer Doudna and Emmanuelle Charpentier saw how humans could harness them.
Basically, it makes it possible to cut DNA at very precise spots and, if desired, take out one stretch of DNA and substitute another. DNA editing had been done in laboratories for about 40 years, but CRISPR/Cas9 is at least 10 times more accurate, faster, easier and less expensive. In about three years, thousands of laboratories around the world have begun to use it successfully in research on an enormous range of living things.
Source: Business Insider
CRISPR is like the Model T Ford. The Model T was not the first car, and it was not the last car, but it was (at least in the United States) the inflection point. Cars went from being the expensive, unreliable province of the curious rich to something anyone could, and soon did, own. With CRISPR/Cas9, and its inevitable descendants, genome editing will become similarly widely available and widely used – “democratized”.
The designer babies myth
Since March 2015, discussion has raged about CRISPR/Cas9 and, more broadly, genome editing. Its focus has been almost exclusively on the use of the technique in people; the early December International Summit on Human Gene Editing was, as its name indicated, devoted entirely to uses in humans.
This is a mistake. Humans will not be early targets. We are terrible experimental animals – we don’t follow orders well, plus we have long generation times … and lawyers. Gene editing in humans for disease or enhancement will need medical professionals and is heavily regulated. Gene editing to make “designer babies” will be insanely risky for many years, would require doctors and in vitro fertilization clinics, is illegal in many countries and, if allowed at all, will be even more heavily regulated. Plus, apart from a few diseases, we know next to nothing about how to “improve” the human genome. Whether designer babies should ever be allowed is deeply controversial; it should not be controversial that any plausible efforts are decades away.
By contrast, consider the non-human world. Few non-human organisms have lawyers or regulators, deeply concerned about their fates. A process that harmed one baby in a hundred would be viewed as terribly risky; one that harmed 99 mosquito larvae out of 100 would not be noticed. And, unlike treating human disease or making human babies, no doctors or clinics are needed – given the ease of CRISPR, a smart undergraduate with a few hundred dollars and a garage could modify many non-human organisms: animal, plant or bacterial.
Do your own gene-editing
Researchers around the world, academic and corporate, have been working on genetic modifications to plants, animals and microbes for agriculture or biofuel purposes for years. CRISPR/Cas9 does not mean that they will suddenly succeed, but it improves their odds. More importantly, CRISPR/Cas9 makes it possible for far more laboratories – or even “do it yourself biohackers” – to become involved. (Already, someone has raised more than $25,000 over the internet to develop do-it-yourself CRISPR kits for resale to interested amateurs.) The world is split over the acceptability of genetically modified crops; to what extent that split will continue if CRISPR/Cas9 brings more drought-resistant, heat-resistant or salt-resistant crops, remains to be seen.
Crops and fuel are the “big ticket” items for DNA modification, but spare some thought for other uses. Pets, for example. Look what we’ve done to wolves in the last few thousand years: turned them into everything from St. Bernards to chihuahuas. BGI, a Chinese genomics firm, is already selling miniature pigs modified through CRISPR/Cas9 as pets.
Or gardens. Gardeners are always trying to create new varieties, including the elusive blue rose. CRISPR/Cas9 will be a powerful help.
Bring back the dragons
Enthusiasts for “de-extinction” (bringing back extinct species or close proxies of them) are already using CRISPR/Cas9 in preliminary work. Artists, seeking to make truly living art, will not be far behind. Neither will those who, for whatever reason, want to make fantastic animals. I expect dragons and unicorns, or their more or less reasonable facsimiles, before mid-century.
CRISPR/Cas9 can be used more seriously to diminish, or perhaps eliminate, infectious diseases spread by animal vectors. Two different gene-editing strategies against mosquito-borne diseases are already in research or development. One, already in field trials, cuts down populations of particular species of mosquitos by making offspring sterile; the other makes the mosquito inhospitable to pathogens, like the malarial plasmodium, that go on to infect humans. No mosquitos carrying malaria eventually means no humans with malaria.
Most seriously, though, CRISPR/Cas9 does not just help those with benign intentions. Creating a dangerous pathogen for biological warfare also becomes easier. Modifying the normal human gut microbe E. coli (the DNA sequence of which is well known) into one of several disease-causing variants (whose sequences are also known) may become, literally, child’s play.
A riskier future
Many of these non-human uses take on greater significance in light of a related development called “gene drive”. It speeds up the passage of a modified gene through a wild population of, say, mosquitos by using CRISPR/Cas9 not only to modify the first generation, but by having the same CRISPR/Cas9 complex stay active in all subsequent generations. This converts the “normal” genes inherited from an unmodified mosquito parent into the desired modified form. As a result, the modified gene might spread through the entire population very quickly, perhaps before anyone even knows what is happening.
A world where an academic lab, a business or a guy in a garage can quickly change the DNA of an entire species is worrisome. Worse is the fact that current national regulatory structures are not set up to deal with CRISPR/Cas9. The US federal government regulates some genetically modified organisms through old statutes implemented in different parts by the Department of Agriculture, the Food and Drug Administration, and the Environmental Protection Agency – but these statutes will not fit all that CRISPR/Cas9 can accomplish.
Worse still, although a new, more appropriate regulatory approach might be able to limit unstudied and potentially dangerous non-human uses of CRISPR/Cas9 by universities or industries, how will it govern (or even find out about) small do-it-yourself labs, whether run by well-intentioned biohackers or bioterrorists?
How to regulate non-human uses of CRISPR/Cas9 poses serious challenges that will take substantial attention, and, ultimately political will. Instead, we see lengthy discussion of, and occasional legislative grandstanding about, human uses that are much better regulated, much more easily contained, and much further in the future. We need to be much less parochial – and we needed to start yesterday.
Author: Henry T "Hank" Greely is the Deane F. and Kate Edelman Johnson Professor of Law and Professor, by courtesy, of Genetics at Stanford University. He is participating in the World Economic Forum’s Annual Meeting in Davos.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
Forum Stories newsletter
Bringing you weekly curated insights and analysis on the global issues that matter.
More on Emerging TechnologiesSee all
Shahid Ahmed
April 23, 2025
Manfred Elsig and Rodrigo Polanco Lazo
April 23, 2025
Michael Siegel
April 23, 2025
Khalid Alaamer
April 22, 2025
Gustavo Maia
April 22, 2025
Cathy Hackl
April 21, 2025





